Current Projects:
Theoretical and computational work on partitioning concept:
Since recently, we are dealing with modeling of thermoresponsive polymers. Our particular interest exist in the effect of salts on stability of biomolecules = (bio)polymers, and proteins. Apart from qualitative analysis (i.e., ordering of salts into series), the quantitative analysis (how strong are the effects) is employed via the so called Kirkwood-Buff theory.
Tunning of Lower Critical Solution Temperature (LCST):
- We are working on novel way of interpretation of LCST data of PNIPAM in salt solutions. link
- LCST can be now predicted for weakly charged copolymer in salt solutions, if the thermodynamics for reference polymer is well known. Our simple theory with only two global fit parameters provided excellent fit of experimental data in concentration and charge fraction domain. link
Modeling of polymer collapse, pearl-neckless structures, and role of cosolute:
- Employing Brownian dynamics (solvent as viscous medium), we have investigated the behavior of long polymer chain under the action of cosolvent, varying the interaction strength. Three regimes were observed: collapsed due to cosolvent exclusion, swollen due to weak cosolvent attraction, and collapsed due to bridging cosolvent. link
Past Projects:
Electrophoresis:
- Electrophoretic separation of neutral compounds. link
- Overcharging in PRL; oligoglutamic acid travel as cation in presence of even milimolar concentration of trivalent cations! link
- Guanidinium cation stacks with guanidinium moieties of tetraarginine sidechains. This effect was quantified in electrophoretic mobility measurements. link
- Beyond Onsager-Fuoss description in electrophoretic mobility evaluation. We have employed Bjerrum description to account for strong electrostatic-based binding of polyelectrolytes with multiply charged ions. link
Interaction of ions with functional groups:
- Oligoglycine solubility changes in salt solutions are crucially dependent on protection of terminal groups. Fourty years old experimentally measurement were most likely done on one-side terminated compounds, not on both-sides terminated are assumed. As these results were traditionally interpreted as interaction of ions with peptide bond, one has to be careful in application of subsequently developed models. link1, link2
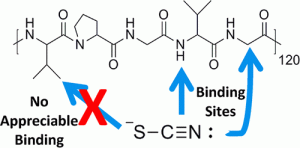
- Chaotropes (like SCN-) bind only to certain methyl moieties in proteins. Applying Kirkwood-Buff theory of preferential binding form simulation side, and measuring chemical shifts in NMR, we have shown that the binding of large anions (I-, SCN- ) to methyl group depends on its environment. In particular, in purely hydrophobic environment (isopropyl moiety) results in no binding, while in amide-bond environment the binding is very strong. link
Interaction of ions and osmolytes with biomolecules:
- For HIV protease and LinB enzyme the enzymatic activity was found to be sensitive to the addition of salt. The viable mechanism of salt action was presented. HIV1, HIV2, LinB
- Denaturant-specific unfolding pathways, were described for TrpCage minipeptide. Originating from different affinity to backbone and sidechain moieties. link
Ions at air/water interfaces:
- Guanidinium has unusual pattern at air/water interface. It is overall weakly depleted from the interface, but probability of its parallel orientations are significantly enhanced. link1, (experimetal collaboration is submitted)
- The peculiar behavior of mixes salt solutions (such as NaCl/NaI). One salt is salt-in or out by the other salt. link
Misterious guanidinium:






Pingback: I moved to ICT Prague | Jan Heyda
Pingback: Let me introduce myself … | Jan Heyda
Pingback: Colorful effects of guanidinium salts on biomolecules make it to JACS | Computer modeling of complex systems